What is the best antibiotic for skin infections
Current Treatment Options for Acute Skin and Skin-structure Infections
Clin Infect Dis. 2019 Apr 1; 68(Suppl 3): S206S212.
Current Treatment Options for Acute Skin and Skin-structure Infections
Yoav Golan
Department of Internal Medicine, Division of Infectious Diseases, Tufts Medical Center, Boston, Massachusetts
Department of Internal Medicine, Division of Infectious Diseases, Tufts Medical Center, Boston, Massachusetts
CopyrightThe Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (
http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial re-use, please contact [email protected]
Abstract
Acute bacterial skin and skin-structure infections (ABSSSIs) are a common reason for seeking care at acute healthcare facilities, including emergency departments. Staphylococcus aureus is the most common organism associated with these infections, and the emergence of community-associated methicillin-resistant Staphylococcus aureus (MRSA) has represented a considerable challenge in their treatment. To address this need, a number of new antibiotics have been developed for the treatment of ABSSSIs in the past several years. Most of these agents focus primarily on gram-positive organisms, particularly MRSA; however, there has not been an oral agent that can reliably treat MRSA, as well as relevant gram-negative pathogens. Acute skin infections that involve mixed gram-positive and gram-negative pathogens must also be considered as they can be associated with discordant antimicrobial therapy. Here, I review ABSSSI treatment guidelines in the hospital setting and discuss current and future antibiotic options for treatment of this commonly encountered infection.
Keywords: skin infection, abscess, antibiotic resistance, therapy
Acute bacterial skin and skin-structure infections (ABSSSIs) are commonly encountered infections in various healthcare settings [1]. Over the last 2 decades, community-associated methicillin-resistant Staphylococcus aureus (MRSA) has emerged as the most common cause of purulent skin infections in the United States with accompanying higher rates of complications (eg, abscess), recurrence, and treatment failures, often leading to hospitalization [2]. The overall burden of managing such common infections has resulted in added healthcare costs [2].
The US Food and Drug Administration (FDA) definition of ABSSSI includes cellulitis/erysipelas, major skin abscesses, and wound infections, with all requiring a minimum lesion surface area of 75 cm2 () [1, 3, 4]. ABSSSI is a common reason for patients seeking care in various healthcare settings, including emergency departments [5]. Most are treated effectively as outpatients [1]. The decision-making process for admitting patients to the hospital for ABSSSI is complex and often very subjective. In general, patients with all of the following characteristics can be managed as outpatients: no signs of sepsis, low suspicion of deep soft tissue infection including necrotizing fasciitis, and lack of exacerbation of comorbidities. For the majority of patients, empiric treatment is most often directed against gram-positive cocci [1, 6].
Table 1.
US Food and Drug Administration Definition of Acute Bacterial Skin and Structure Infection [1, 4]
| Conditions Included in the Definition | Conditions Not Included in the Definition |
|---|---|
| Cellulitis/erysipelas | Impetigo and minor cutaneous abscess |
| Wound infections | Animal or human bites |
| Major cutaneous abscess | Necrotizing fasciitis |
| Diabetic foot infection | |
| Burns | |
| Chronic wound infection | |
| Myonecrosis | |
| Ecthyma gangrenosum |
During hospitalization, healthcare providers may need to reevaluate antimicrobial treatment and the need for surgical intervention [1]. Antimicrobial therapy may need to be adjusted, particularly in the elderly due to comorbidities such as renal insufficiency. Once culture and susceptibility results are available, if indicated, deescalation of antimicrobial therapy is recommended. Of note, the role of MRSA in cellulitis without a wound or purulence is not as clear due to the absence of culturable material.
Here, I review ABSSSI treatment guidelines in the hospital setting and discuss current and future antibiotic options for treatment of this commonly encountered infection. Although many of the studies discussed here have used different terms to describe these infections, such as complicated skin and skin-structure infections or complicated skin and soft-tissue infections, throughout the article I will refer to them as ABSSSI.
TREATMENT GUIDELINES
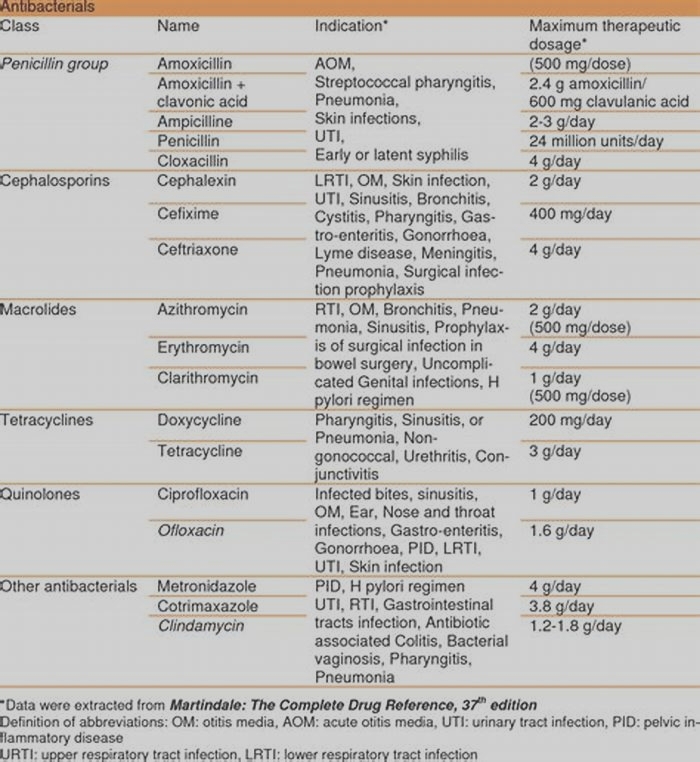
Most recently, the Infectious Diseases Society of America (IDSA) published practice guidelines for the diagnosis and management of ABSSSIs [6]. This publication predates the FDA approval of several newer antibiotics for the treatment of ABSSSIs, including dalbavancin, oritavancin, tedizolid, and delafloxacin. The guidelines divide ABSSSIs into purulent and nonpurulent categories. Cellulitis and erysipelas are considered nonpurulent, while abscesses are categorized as purulent. Vancomycin, linezolid, tigecycline, daptomycin, ceftaroline, and telavancin are all considered appropriate antimicrobial agents for treatment of severe purulent infections, while trimethoprim-sulfamethoxazole and doxycycline are recommended for moderate purulent infections. For methicillin-susceptible S. aureus (MSSA), cefazolin and clindamycin are recommended for severe infections and dicloxacillin and cephalexin for moderate infections [6]. Vancomycin plus piperacillin/tazobactam is recommended as a first-line treatment option for severe nonpurulent infections, particularly suspected necrotizing or polymicrobial infections. However, this combination has been associated with an increased risk of acute kidney injury when compared with vancomycin with or without other beta-lactams [7]. Options for treatment of mild to moderate nonpurulent infections include cefazolin, ceftriaxone, clindamycin, and penicillin [6]. Although there is a lack of data to support the optimal duration of antibiotic therapy, the guidelines recommend a treatment duration of 710 days [6].
Recent antibiotic development for ABSSSIs has focused largely on the coverage of gram-positive organisms, especially MRSA () [812]. However, acute skin infections that involve gram-negative pathogens are associated with a potential risk for inadequate antimicrobial therapy. Clinicians need to consider risk factors for infections due to gram-negative pathogens in selected patients [1]. Moreover, although gram-negative pathogens are commonly found in polymicrobial infections, they have also been observed in monomicrobial skin infections [13, 14]. Cultures with only gram-negative pathogens have been reported in approximately 13% of patients hospitalized with serious skin infections (not limited to ABSSSI), while mixed cultures have been found in 11%21% [15, 16]. Selection of empiric antibiotic therapy for suspected gram-negative skin infections should be guided by local epidemiologic patterns, as well as infection type and individual patient characteristics [1, 8].
Table 2.
Summary of Recently Approved Antibiotics for the Treatment of Acute Bacterial Skin and Structure Infection [812]
| Product Characteristics | Dalbavancin | Oritavancin | Tedizolid | Delafloxacin |
|---|---|---|---|---|
| Indications | ABSSSI caused by susceptible strains of gram-positive microorganisms | ABSSSI caused by susceptible strains of gram-positive microorganisms | ABSSSI caused by susceptible strains of gram-positive microorganisms | ABSSSI caused by susceptible strains of gram-positive and gram-negative microorganisms |
| Microbiology | In vitro and clinical activity against the following aerobic and facultative gram-positive bacteria: Staphylococcus aureus (including MRSA) Streptococcus pyogenes Streptococcus agalactiae Staphylococcus dysgalactiae Staphylococcus anginosus group (including Staphylococcus anginosus, Staphylococcus intermedius, Staphylococcus constellatus) Enterococcus faecalis (vancomycin- susceptible isolates only) | In vitro and clinical activity against: S. aureus, (including MRSA) S. pyogenes S. agalactiae S. anginosus group (including S. anginosus, S. intermedius, S. constellatus) E. faecalis | In vitro and clinical activity against gram- positive bacteria including: S. aureus (including MRSA) S. agalactiae S. anginosus group (including S. anginosus, S. intermedius, S. constellatus) S. dysgalactiae S. pyogenes E. faecalis (vancomycin-susceptible isolates only) | In vitro and clinical activity against the following aerobic gram-positive and gram-negative bacteria: S. aureus (including MRSA) Staphylococcus haemolyticus Staphylococcus lugdunensis S. pyogenes S. agalactiae S. dysgalactiae S. anginosus group (including S. anginosus, S. intermedius, S. constellatus) E. faecalis Escherichia coli Klebsiella pneumonia Enterobacter cloacae Pseudomonas aeruginosa |
| Formulations | IV | IV | IV/oral | IV/oral |
| Dosing | Single-dose regimen: 1500 mg IV over 30 min Two-dose regimen: 1000 mg IV over 30 min followed 1 week later by 500 mg IV over 30 min | Single-dose regimen: 1200 mg IV over 3 hours | 200 mg IV/oral tablet daily for 6 days; IV infusion over 1 hour | 300 mg IV over 60 min every 12 hours for 514 days OR 450 mg oral tablet every 12 hours for 514 days |
The emergence of community-associated MRSA strains has greatly influenced the selection of empirical antibiotic therapy for ABSSSIs. To provide adequate empirical coverage for MRSA, an understanding of local microbial epidemiologic patterns and susceptibility patterns is essential [3]. For the treatment of an abscess, the IDSA guidelines recommend, in addition to incision and drainage, administration of an antibiotic active against MRSA when the initial antibiotic treatment has failed or when the patient has immunosuppression, systemic inflammatory response syndrome (SIRS), or hypotension [6]. For cellulitis and erysipelas, including an antibiotic with activity against MRSA and group A streptococcus is recommended when the infection is associated with penetrating trauma or when there is evidence of MRSA infection, MRSA colonization, injecting drug use, or SIRS [6]. Further, including an antimicrobial agent active against MRSA in the treatment of surgical wound infections is recommended in patients with risk factors for infection by this microorganism (nasal colonization or prior infection, hospitalization, or recent antibiotic administration) [6]. Anti-MRSA agents for ABSSSIs include vancomycin, which is considered the first-line parenteral treatment of serious MRSA infections in hospitalized patients, as well as linezolid, daptomycin, and tigecycline () [6]. Other agents with reliable MRSA activity that have been approved for ABSSSIs include ceftaroline, tedizolid, dalbavancin, oritavancin, and telavancin. Additional factors for administering these medications include tolerability, formulation, cost, and dosing limitations associated with these agents, such as vancomycin-related nephrotoxicity and linezolid-associated myelosuppression.
Table 3.
2014 Infectious Diseases Society of America Recommendations for Antibiotic Treatment of Acute Bacterial Skin and Structure Infection Caused by Methicillin-resistant Staphylococcus aureus [6]
| Antibiotic | Route | Recommended Dosing in Adults |
|---|---|---|
| Vancomycin | IV | 15 mg/kg every 12 hours |
| Linezolid | IV/oral | IV: 600 mg every 12 hours Oral: 600 mg twice a day |
| Clindamycin | IV/oral | IV: 600 mg every 8 hours Oral: 300450 mg 4 times a day |
| Daptomycin | IV | 4 mg/kg daily |
| Ceftaroline | IV | 600 mg every 12 hours |
| Doxycycline, minocycline | Oral | 100 mg twice a day |
| Trimethoprim-sulfamethoxazole | Oral | 12 double strength tablets twice a day |
ANTIBIOTIC TREATMENT OPTIONS FOR ABSSSI
Beta-lactams
Among the beta-lactams, ceftaroline fosamil is an option for the initial empirical treatment of patients hospitalized with ABSSSIs, including those with suspected MRSA infection. Ceftaroline, administered twice daily, is an advanced-generation, intravenous (IV), bactericidal cephalosporin with broad-spectrum activity against gram-positive bacteria, including MRSA and some gram-negative bacteria, with the exception of Pseudomonas aeruginosa [17]. In 2 phase 3 studies [18, 19], ceftaroline showed noninferiority to vancomycin plus aztreonam in hospitalized patients with ABSSSI. Diarrhea was the most commonly reported adverse event (AE), and there was a low incidence of Clostridium difficile-associated diarrhea [18, 19]. Ceftaroline has also demonstrated a low potential for the selection of in vitro resistance for drug-resistant gram-positive organisms, including MRSA [17]. There is no oral formulation of ceftaroline.
Cyclic Lipopeptides
Daptomycin is a cyclic lipopeptide that is dosed once daily and exhibits rapid, concentration-dependent bactericidal activity against a broad range of gram-positive pathogens, including MRSA and vancomycin-resistant pathogens [20]. In 2 trials, the clinical success rate for daptomycin in patients with ABSSSIs was 83.4% with a shorter duration of treatment than comparator antibiotics [21]. A randomized, controlled trial demonstrated that in patients admitted to an observation unit with ABSSSI, IV daptomycin was noninferior to vancomycin for the primary endpoint of objective discharge criteria with no change in antibiotic therapy or return to the emergency department for the same cellulitis within 30 days of discharge [22]. The most notable side effect of daptomycin is myotoxicity, which is reversible upon discontinuation of therapy [20].
Fluoroquinolones
Fluoroquinolones such as ciprofloxacin, levofloxacin, and moxifloxacin are not commonly utilized as treatment agents for ABSSSIs caused by MRSA and are not endorsed in the IDSA guidelines as such. This is primarily due to decreased susceptibility of MRSA to fluoroquinolones, which highlights the need for additional treatment options for skin infections that are caused by fluoroquinolone-resistant organisms [23]. Delafloxacin is a novel non-zwitterionic fluoroquinolone approved by the FDA in 2017 for ABSSSIs [8]. Delafloxacin has broad-spectrum activity that allows for potential use in infections caused by gram-positive pathogens, including MRSA and many gram-negative pathogens, without the need for combination therapy [8, 23]. Delafloxacin can be administered IV or orally [23]. The chemical profile of delafloxacin is unique among quinolones, which makes it particularly active in acidic environments [24, 25]. Delafloxacin demonstrates greater in vitro and in vivo activity against the majority of gram-positive pathogens compared with levofloxacin, including MRSA isolates and isolates not susceptible to levofloxacin [23, 26].
Two phase 3, randomized, double-blind, active-controlled studies demonstrated that delafloxacin was noninferior to the combination of vancomycin plus aztreonam for the treatment of ABSSSIs [8, 27]. These trials compared delafloxacin with vancomycin plus aztreonam for a period of 514 days. The primary efficacy endpoint of objective response rate 4872 hours after treatment initiation was 78.2% in the delafloxacin arm and 80.9% in the vancomycin/aztreonam arm (mean treatment difference, 2.6%; 95% confidence interval [CI], 8.78% to 3.57%) in the first trial [8] and 83.7% and 80.6% (mean treatment difference, 3.1%; 95% CI, 2.0% to 8.3%) in the second trial [27]. Cure rates in those with MRSA infection were similar between the delafloxacin group (100%) and the vancomycin/aztreonam group (98.5%). The rate of treatment-emergent AEs was similar in both arms. AEs that resulted in treatment discontinuation were higher in the vancomycin plus aztreonam group compared with the delafloxacin group (4.3% vs 0.9%) [8].
Several studies have demonstrated minimal potential for drugdrug interactions with delafloxacin, as well as no evidence of QT interval prolongation or phototoxicity [2831]. There is no apparent effect of food or age on delafloxacin pharmacokinetics, and weight-based dosing and drug monitoring are not required.
Glycopeptides
The IDSA guidelines recommend vancomycin as a first-line agent for ABSSSIs caused by MRSA [6]. Nephrotoxicity is the most serious AE associated with vancomycin, and risk factors can include the higher doses and prolonged treatment, high serum trough concentrations, and concurrent administration of nephrotoxic agents [32]. The combination of vancomycin with piperacillin-tazobactam, which is frequently used to cover relevant gram-negative pathogens, has been shown to be associated with acute kidney injury [7]. Vancomycin-associated nephrotoxicity has been linked to longer hospital stays, increased medical costs, need for additional antibiotics, and, in rare cases, dialysis [32]. Since elevated trough concentrations of vancomycin are associated with a higher incidence of nephrotoxicity, careful monitoring of these concentrations is required. Consensus guidelines for vancomycin therapeutic monitoring recommend targeting trough concentrations of 1520 mg/L to limit nephrotoxicity. In addition, concentrations 10 mg/L are recommended to prevent development of bacterial strains resistant to vancomycin [33, 34]. Vancomycin-resistant Enterococcus (VRE) species have been reported in monomicrobial ABSSSIs [35], and vancomycin-intermediate and vancomycin-resistant S. aureus also present treatment challenges.
Telavancin is another member of the lipoglycopeptide class and a semisynthetic derivative of vancomycin [36]. It exhibits activity against a broad range of gram-positive organisms, including S. aureus, coagulase-negative staphylococci, and Streptococcus species. It has activity against MRSA, vancomycin-intermediate S. aureus, vancomycin-susceptible and VRE species, and various other gram-positive anaerobic organisms [36]. In a metaanalysis of randomized trials, telavancin showed higher eradication rates (odds ratio [OR] = 1.71 [1.082.70]) and a trend toward better clinical response compared with vancomycin (OR = 1.55 [0.932.58]) [36]. This analysis also showed that telavancin was associated with higher rates of serious AEs, the most common of which were renal in nature.
Glycylcyclines
Tigecycline is an IV broad-spectrum glycylcycline that exhibits in vitro activity against gram-positive, gram-negative, anaerobic, and atypical organisms and some multidrug-resistant pathogens [37]. It is unaffected by standard tetracycline resistance mechanisms and has not shown any cross-resistance with common resistance mechanisms seen with other antibiotic classes, although resistance through specific efflux pump mechanisms has been reported [37]. Two randomized, double-blind trials found tigecycline to be noninferior to the combination of vancomycin and aztreonam in adults for the treatment of ABSSSIs [38, 39], and a more recent randomized study showed it to be noninferior to ampicillin/sulbactam or amoxicillin-clavulanate with or without vancomycin in the treatment of ABSSSIs [37]. The most commonly observed AEs are nausea and vomiting. Cases of acute pancreatitis following tigecycline administration have been observed [40].
Lipoglycopeptides
The lipoglycopeptide class is comprised of 2 IV options: dalbavancin and oritavancin. Dalbavancin has activity against most gram-positive pathogens and possesses a long plasma half-life (610 days) [41]. A 2-dose regimen of IV dalbavancin, given on day 1 (1 gram) and day 8 (0.5 gram), has been shown to be noninferior to twice-daily vancomycin followed by oral linezolid for the treatment of ABSSSI, with less frequent AEs [42]. The most common treatment-related AEs in either group were nausea, diarrhea, and pruritis [42]. Additionally, a single-dose infusion of dalbavancin has shown to have noninferior efficacy to the 2-dose dalbavancin regimen with respect to early clinical response, with a similar safety profile [43].
Oritavancin has activity against gram-positive organisms, and its long elimination half-life allows for single-dose treatment [44]. The safety of oritavancin was evaluated in 2 phase 3 studies enrolling 976 oritavancin patients and 983 vancomycin patients [44]. From the safety database, the incidences of AEs, serious AEs, and discontinuations due to AEs were similar for oritavancin and vancomycin. The most frequently reported AEs were nausea, headache, and vomiting [44]. Single-dose oritavancin has shown similar efficacy to that of twice-daily vancomycin at both early clinical and end-of-therapy time points [45]. Oritavancin also has activity against vanA-mediated VRE species [46].
Oxazolidinones
Linezolid is available in IV and oral formulations [47]. Linezolid provides an option for early switch to oral therapy, and dose reduction is not necessary in patients with renal insufficiency [40]. Linezolid has shown to be active against many resistant gram-positive organisms including MRSA, VRE, and macrolide-resistant streptococci. In a study of patients with ABSSSIs due to MRSA, compared with vancomycin, linezolid-treated patients had significantly higher clinical and microbiological success rates at the end of treatment [48]. Linezolid-associated thrombocytopenia and anemia are typically mild, reversible, and duration dependent [49]. There is an interaction between linezolid and selective serotonin reuptake inhibitors.
Tedizolid, a bacterial protein synthesis inhibitor, can be administered once daily, either orally or IV at equivalent doses [50]. In 2 randomized trials, treatment with tedizolid for 6 days was shown to be noninferior to linezolid for 10 days [51, 52]. In addition, differences in the incidence of gastrointestinal AEs have been observed between tedizolid and linezolid [5052], possibly due to the effects of these agents on intestinal flora [50]. The effect of tedizolid on normal intestinal flora has been shown to be limited and reversible [53], and another study showed that linezolid led to marked changes in gut flora composition [54]. Further, the less frequent dosing with tedizolid (once daily vs twice daily with linezolid) may also somewhat reduce the overall risk of developing gastrointestinal AEs [50].
Trimethoprim-sulfamethoxazole and Clindamycin
Several recent trials have evaluated the efficacy and safety of trimethoprim-sulfamethoxazole and clindamycin for the treatment of uncomplicated ABSSSIs [5557]. In a study of uncomplicated cutaneous abscesses treated with drainage, adjunctive treatment with trimethoprim-sulfamethoxazole for 7 days resulted in higher cure rates compared with placebo. In another randomized study of uncomplicated wound infections, trimethoprim-sulfamethoxazole and clindamycin were similarly effective with comparable cure rates and AEs [56]. A randomized clinical trial involving adults and children found no differences between clindamycin and trimethoprim-sulfamethoxazole in efficacy or safety for the treatment of uncomplicated skin infections. However, the development of new infections by 1 month of follow-up was lower in the clindamycin-treated group [57]. Neither of these 2 antibiotics has been evaluated in a head-to-head comparison with the newer MRSA-active antibiotics.
INVESTIGATIONAL AND RECENTLY APPROVED ANTIBIOTICS FOR ABSSSI
Aminomethylcyclines: Omadacycline
Omadacycline is a first-in-class broad-spectrum aminomethylcycline [58]. Similar to tetracyclines, omadacycline binds to the 30S ribosomal subunit of target gram-positive and gram-negative bacteria, resulting in inhibition of protein synthesis [58]. Omadacycline has high potency against MRSA and MSSA and maintains activity in the presence of ribosomal protection and efflux tetracycline resistance genes. It has proven activity against tetracycline-resistant pathogens and is not affected by mechanisms of resistance to other classes of antibacterial agents [58]. The pharmacologic properties of omadacycline allow for IV or oral administration with once-daily dosing. Omadacycline was shown to be well tolerated and effective for the treatment of complicated skin infections in a phase 2, randomized, investigator-blind, multicenter trial [59]. There were fewer treatment-related AEs in omadacycline-treated patients (21%) compared with the linezolid group (30.6%), the most common of which were GI related (18.9% omadacycline, 18.5% linezolid).
Pleuromutilins: Lefamulin
Lefamulin is currently in late stage development as an IV and oral treatment for ABSSSIs [60]. Lefamulin inhibits protein synthesis by binding to the 50S ribosomal subunit [61]. It exhibits activity against frequently identified gram-positive skin pathogens, including MRSA, MSSA, Streptococcus pyogenes, Streptococcus agalactiae, and vancomycin-resistant Enterococcus faecium [62].
Pleuromutilins are not affected by resistance mechanisms involving major antibiotic classes, including fluoroquinolones and tetracyclines [60]. A phase 2 proof-of-concept study evaluated the use of lefamulin vs vancomycin in the treatment of patients with ABSSSIs caused by a gram-positive pathogen. In that trial, 207 patients were randomized to IV lefamulin 100 mg, lefamulin 150 mg, or vancomycin 1 g every 12 hours. Clinical success rates at test-of-cure in the clinically evaluable population were comparable across all 3 groups: 90% lefamulin 100 mg group; 88.9% lefamulin 150 mg group, and 92.2% vancomycin group. In addition, clinical response rates at day 3 were similar across the 3 treatment groups. The incidence of drug-related AEs was lower for lefamulin (34.3% and 39.4% in the 100 mg and 150 mg groups, respectively) compared with vancomycin (53.0%) [62].
CONCLUSIONS
ABSSSIs pose a significant burden to the healthcare system. The challenge of treating these infections has been lessened by recent development of new antibiotic treatment options that target gram-positive organisms including MRSA strains. However, new antibiotics that can cover both gram-positive pathogens (including MRSA) and gram-negative pathogens can fulfill an important need in the treatment of selected hospitalized patients with acute skin infections. Examples of such infections include abscesses with mixed bacterial growth, surgical-site infections, particularly those related to abdominal surgery, and selected acute skin infections in patients with diabetes. Furthermore, antibiotics that can offer hospital clinicians other attributes, such as the flexibility to transition from IV to oral formulation, infrequent dosing, pharmacokinetics that are unaffected by various patient characteristics, a low risk of drugdrug interactions, and no requirement for therapeutic monitoring, will be particularly useful when treating ABSSSIs.
Notes
Acknowledgments. The author appreciates the expert feedback provided by Glenn Tillotson of GST Micro LLC. Assistance with the preparation of this manuscript was provided by Prasad Kulkarni and Alexandra Rayser of the HealthCare Alliance Group, Voorhees, New Jersey.
Financial support. Editorial support was funded by Melinta Therapeutics and was provided by HealthCare Alliance Group.
Supplement sponsorship. This supplement is sponsored by Melinta Therapeutics, Inc.
Potential conflicts of interest. Y. G. has received speaker fees from Merck, Pfizer, Allergan, Melinta Pharmaceuticals, Tetraphase, and Achaogen; research funding from Allergan and Merck; and advisor fees from Merck, Achaogen, Tetraphase, Paratek, Melinta, and Shionogi unrelated to this manuscript. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
1.
Russo A, Concia E, Cristini F, et al.. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect 2016; 22 Suppl 2:S2736. [PubMed] [Google Scholar]2.
Pollack CV Jr, Amin A, Ford WT Jr, et al.. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med 2015; 48:50819. [PubMed] [Google Scholar]3.
Esposito S, Bassetti M, Concia E, et al.; Italian Society of Infectious and Tropical Diseases Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: an update. J Chemother 2017; 29:197214. [PubMed] [Google Scholar]5.
Abrahamian FM, Talan DA, Moran GJ. Management of skin and soft-tissue infections in the emergency department. Infect Dis Clin North Am 2008; 22:89116, vi. [PubMed] [Google Scholar]6.
Stevens DL, Bisno AL, Chambers HF, et al.; Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e1052. [PubMed] [Google Scholar]7.
Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med 2018; 46:1220. [PubMed] [Google Scholar]8.
Pullman J, Gardovskis J, Farley B, et al.; PROCEED Study Group Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a phase 3, double-blind, randomized study. J Antimicrob Chemother 2017; 72:347180. [PMC free article] [PubMed] [Google Scholar]9.
Baxdela (Delafloxacin) for injection and tablets [package insert]. Lincolnshire, IL: Melinta Therapeutics, 2017. [Google Scholar]10.
Dalvance (dalbavancin) for injection [package insert]. Irvine, CA: Allergan, 2016. [Google Scholar]11.
Orbactiv (oritavancin) for injection [package insert]. Parsippany, NJ: The Medicines Company, 2016. [Google Scholar]12.
Sivextro (tedizolid) for injection and tablets [package insert]. Whitehouse Station, NJ: Merck & Co., 2016. [Google Scholar]13.
Bassetti M, Merelli M, Temperoni C, Astilean A. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob 2013; 12:22. [PMC free article] [PubMed] [Google Scholar]14.
Itani KM, Merchant S, Lin SJ, et al.. Outcomes and management costs in patients hospitalized for skin and skin-structure infections. Am J Infect Control 2011; 30:429. [PubMed] [Google Scholar]15.
Berger A, Oster G, Edelsberg J, Huang X, Weber DJ. Initial treatment failure in patients with complicated skin and skin structure infections. Surg Infect (Larchmt) 2013; 14:30412. [PubMed] [Google Scholar]16.
Halilovic J, Heintz BH, Brown J. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect 2012; 65:12834. [PubMed] [Google Scholar]17.
Frampton JE.Ceftaroline fosamil: a review of its use in the treatment of complicated skin and soft tissue infections and community-acquired pneumonia. Drugs 2013; 73:106794. [PubMed] [Google Scholar]18.
Corey GR, Wilcox M, Talbot GH, et al.. CANVAS 1: The first phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010; 65(Suppl 4):4151. [PubMed] [Google Scholar]19.
Wilcox M, Corey GR, Talbot GH, et al.. CANVAS 2: The second phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010; 65(Suppl 4):5365. [PubMed] [Google Scholar]20.
Wang SZ, Hu JT, Zhang C, et al.. The safety and efficacy of daptomycin versus other antibiotics for skin and soft-tissue infections: a meta-analysis of randomised controlled trials. BMJ Open 2014; 4:e004744. [PMC free article] [PubMed] [Google Scholar]21.
Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI; Daptomycin 98-01 and 99-01 Investigators The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 2004; 38:167381. [PubMed] [Google Scholar]22.
Shaw GJ, Meunier JM, Korfhagen J, et al.. Randomized controlled noninferiority trial comparing daptomycin to vancomycin for the treatment of complicated skin and skin structure infections in an observation unit. J Emerg Med 2015; 49:92836. [PubMed] [Google Scholar]23.
McCurdy S, Lawrence L, Quintas M, et al.. In vitro activity of delafloxacin and microbiological response against fluoroquinolone-susceptible and nonsusceptible Staphylococcus aureus isolates from two phase 3 studies of acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2017; 61:18. [PMC free article] [PubMed] [Google Scholar]24.
Lemaire S, Tulkens PM, Van Bambeke F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:64958. [PMC free article] [PubMed] [Google Scholar]25.
Van Bambeke F.Delafloxacin, a non-zwitterionic fluoroquinolone in phase III of clinical development: evaluation of its pharmacology, pharmacokinetics, pharmacodynamics and clinical efficacy. Future Microbiol 2015; 10:111123. [PubMed] [Google Scholar]26.
Oshita Y, Yazaki A. In vitro studies with WQ-3034, a newly synthesized anionic fluoroquinolone. 37th Interscience Conference on Antimicrobial Agents and ChemotherapyToronto, Ontario, Canada, Abstr F-164. Washington, DC: American Society for Microbiology, 1997. [Google Scholar]27.
ORiordan W, McManus A, Teras J, et al.. A comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a phase 3, multinational, double-blind, randomized study. Clin Infect Dis 2018; 67:65766. doi:10.1093/cid/ciy165. [PMC free article] [PubMed] [Google Scholar]28.
Litwin JS, Benedict MS, Thorn MD, Lawrence LE, Cammarata SK, Sun E. A thorough QT study to evaluate the effects of therapeutic and supratherapeutic doses of delafloxacin on cardiac repolarization. Antimicrob Agents Chemother 2015; 59:346973. [PMC free article] [PubMed] [Google Scholar]29.
Hoover R, Hunt T, Benedict M, et al.. Safety, tolerability, and pharmacokinetic properties of intravenous delafloxacin after single and multiple doses in healthy volunteers. Clin Ther 2016; 38:5365. [PubMed] [Google Scholar]30.
Lawrence L, Benedict M, Litwin J, et al.. A thorough phase 1 QTc study of delafloxacin compared with placebo and moxifloxacin (MXF). In: Abstracts of the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA. Abstract A1958. Washington, DC: American Society for Microbiology, 2012. [Google Scholar]31.
Ferguson J, Lawrence L, Paulson S, et al.. Assessment of phototoxicity potential of delafloxacin in healthy male and female subjects: a phase 1 study. In: Abstracts of the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego. Abstract F-1198a. Washington, DC: American Society for Microbiology, 2015. [Google Scholar]33.
Rybak M, Lomaestro B, Rotschafer JC, et al.. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:8298. [PubMed] [Google Scholar]34.
Rybak MJ, Lomaestro BM, Rotschafer JC, et al.. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009; 49:3257. [PubMed] [Google Scholar]35.
Guillamet CV, Kollef MH. How to stratify patients at risk for resistant bugs in skin and soft tissue infections? Curr Opin Infect Dis 2016; 29:11623. [PubMed] [Google Scholar]36.
Polyzos KA, Mavros MN, Vardakas KZ, Makris MC, Rafailidis PI, Falagas ME. Efficacy and safety of telavancin in clinical trials: a systematic review and meta-analysis. PLoS One 2012; 7:e41870. [PMC free article] [PubMed] [Google Scholar]37.
Matthews P, Alpert M, Rahav G, et al.; Tigecycline 900 cSSSI Study Group A randomized trial of tigecycline versus ampicillin-sulbactam or amoxicillin-clavulanate for the treatment of complicated skin and skin structure infections. BMC Infect Dis 2012; 12:297. [PMC free article] [PubMed] [Google Scholar]38.
Breedt J, Teras J, Gardovskis J, et al.; Tigecycline 305 cSSSI Study Group Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother 2005; 49:465866. [PMC free article] [PubMed] [Google Scholar]39.
Sacchidanand S, Penn RL, Embil JM, et al.. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int J Infect Dis 2005; 9:25161. [PubMed] [Google Scholar]40.
Eckmann C, Dryden M. Treatment of complicated skin and soft-tissue infections caused by resistant bacteria: value of linezolid, tigecycline, daptomycin and vancomycin. Eur J Med Res 2010; 15:55463. [PMC free article] [PubMed] [Google Scholar]41.
Seltzer E, Dorr MB, Goldstein BP, Perry M, Dowell JA, Henkel T; Dalbavancin Skin and Soft-Tissue Infection Study Group Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis 2003; 37:1298303. [PubMed] [Google Scholar]42.
Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 2014; 370:216979. [PubMed] [Google Scholar]43.
Dunne MW, Puttagunta S, Giordano P, Krievins D, Zelasky M, Baldassarre J. A randomized clinical trial of single-dose versus weekly dalbavancin for treatment of acute bacterial skin and skin structure infection. Clin Infect Dis 2016; 62:54551. [PMC free article] [PubMed] [Google Scholar]44.
Corey GR, Loutit J, Moeck G, et al.. Single intravenous dose of oritavancin for the treatment of gram-positive acute bacterial skin and skin structure infections: summary of safety from the phase 3 SOLO studies. Antimicrob Agents Chemother 2018; 62:doi: 10.1128/AAC.01919-17. [PMC free article] [PubMed] [CrossRef] [Google Scholar]45.
Corey GR, Good S, Jiang H, et al.; SOLO II Investigators Single-dose oritavancin versus 710 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis 2015; 60:25462. [PubMed] [Google Scholar]46.
Ferrndez O, Urbina O, Grau S. Critical role of tedizolid in the treatment of acute bacterial skin and skin structure infections. Drug Des Devel Ther 2017; 11:6582. [PMC free article] [PubMed] [Google Scholar]47.
Wilcox MH.Update on linezolid: the first oxazolidinone antibiotic. Expert Opin Pharmacother 2005; 6:231526. [PubMed] [Google Scholar]48.
Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg 2010; 199:80416. [PubMed] [Google Scholar]49.
Gerson SL, Kaplan SL, Bruss JB, et al.. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother 2002; 46:27236. [PMC free article] [PubMed] [Google Scholar]50.
Shorr AF, Lodise TP, Corey GR, et al.. Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2015; 59:86471. [PMC free article] [PubMed] [Google Scholar]51.
Prokocimer P, De Anda C, Fang E, Mehra P, Das A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 2013; 309:55969. [PubMed] [Google Scholar]52.
Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2014; 14:696705. [PubMed] [Google Scholar]53.
Tanaka T, Hayashi Y, Okumura K, et al.. Oral bioavailability of tedizolid in healthy Japanese subjects in a phase I study [Abstract P1718]. Poster presented at: European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain, May 1013, 2014. [Google Scholar]54.
Lode H, Von der Hh N, Ziege S, Borner K, Nord CE. Ecological effects of linezolid versus amoxicillin/clavulanic acid on the normal intestinal microflora. Scand J Infect Dis 2001; 33:899903. [PubMed] [Google Scholar]55.
Talan DA, Lovecchio F, Abrahamian FM, et al.. A randomized trial of clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated wound infection. Clin Infect Dis 2016; 62:150513. [PMC free article] [PubMed] [Google Scholar]56.
Talan DA, Mower WR, Krishnadasan A, et al.. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med 2016; 374:82332. [PMC free article] [PubMed] [Google Scholar]57.
Miller LG, Daum RS, Creech CB, et al.; DMID 07-0051 Team Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med 2015; 372:1093103. [PMC free article] [PubMed] [Google Scholar]58.
Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. Activities of omadacycline and comparator agents against Staphylococcus aureus isolates from a surveillance program conducted in North America and Europe. Antimicrob Agents Chemother 2017; 61:e024116. [PMC free article] [PubMed] [Google Scholar]59.
Noel GJ, Draper MP, Hait H, Tanaka SK, Arbeit RD. A randomized, evaluator-blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 2012; 56:56504. [PMC free article] [PubMed] [Google Scholar]60.
Paukner S, Reidl R. Pleuromutilins: potent drugs for resistance bugsmode of action and resistance. Cold Spring Harb Perspect Med 2017; 7:pii: a027110. [PMC free article] [PubMed] [Google Scholar]61.
Abbas M, Paul M, Huttner A. New and improved? A review of novel antibiotics for gram-positive bacteria. Clin Microbiol Infect 2017; 23:697703. [PubMed] [Google Scholar]62.
Prince WT, Ivezic-Schoenfeld Z, Lell C, et al.. Phase II clinical study of BC-3781, a pleuromutilin antibiotic, in treatment of patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2013; 57:208794. [PMC free article] [PubMed] [Google Scholar]